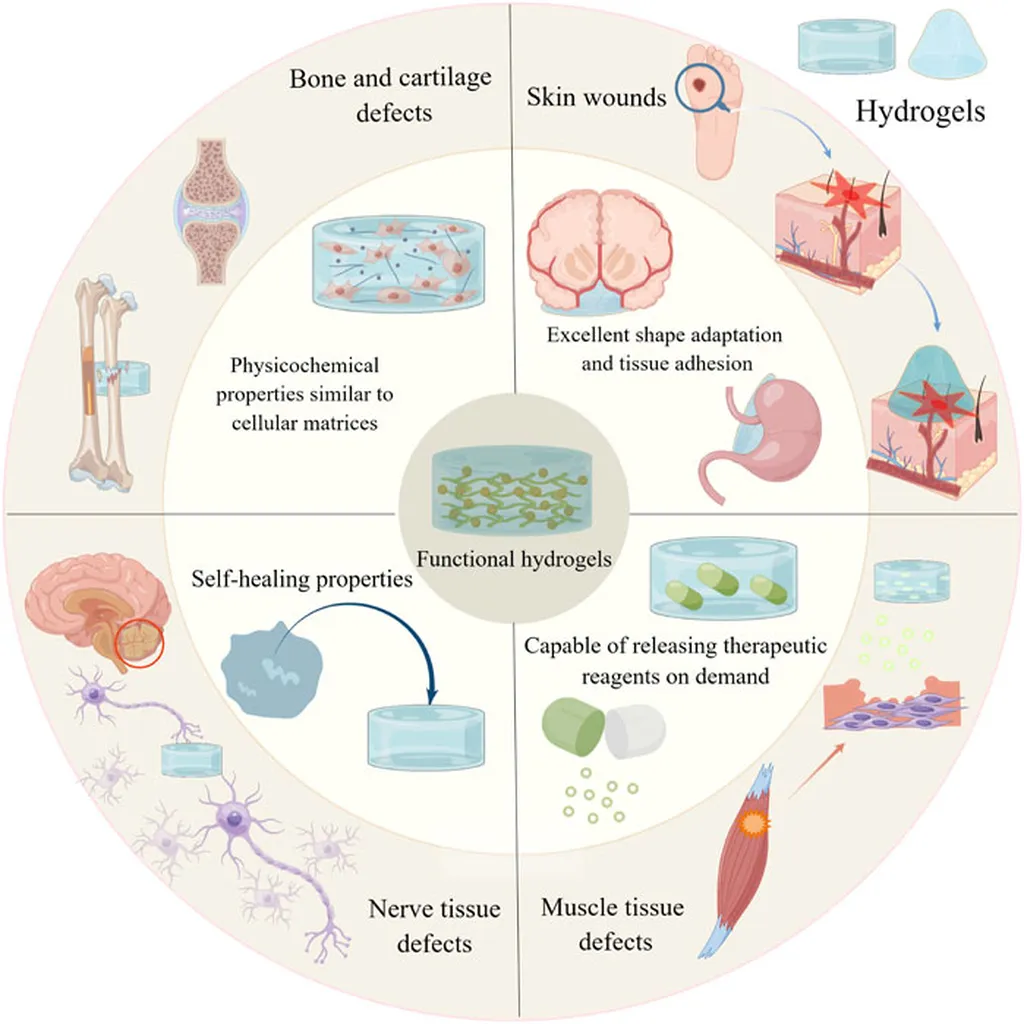
In the ever-evolving landscape of biomedical materials, a groundbreaking study has emerged, promising to revolutionize the way we approach tissue engineering and regenerative medicine. Researchers from the University of Aveiro in Portugal have developed a novel hydrogel system that could significantly impact the repair and regeneration of load-bearing connective tissues, such as cartilage. The study, led by Maria Clara Gomes from the Department of Chemistry and the CICECO-Aveiro Institute of Materials, was recently published in the journal “Materials Research Express,” which translates to “Expressions of Materials Research” in English.
The challenge of creating bioactive hydrogels with mechanical properties akin to those of natural load-bearing tissues has long plagued the biomedical field. Gomes and her team have tackled this issue head-on by incorporating human platelet lysate (PL) proteins, tannic acid (TA), and chitosan methacrylate (CHI-MA) into their hydrogel formulations. The result? A reinforced hydrogel system with mechanical performance that could very well redefine the standards of tissue engineering.
The PL/CHI-MA/TA hydrogels developed by Gomes’ team exhibit exceptional mechanical characteristics, boasting strengths of approximately 7 MPa and stiffness values of 5 MPa. These values are comparable to those found in natural load-bearing tissues like cartilage, making these hydrogels a promising candidate for applications in regenerative medicine.
“The synergistic effect of the PL proteins, tannic acid, and chitosan methacrylate enhances the interaction between the proteins and the polymeric network,” explains Gomes. “This leads to increased strength, toughness, and efficient energy dissipation, which are crucial for load-bearing tissues.”
But the benefits don’t stop at mechanical performance. These hydrogels also support the adhesion and proliferation of human-derived adipose stem cells, further underscoring their potential utility in tissue engineering. The ability to support cell growth is a critical factor in the success of any biomaterial intended for regenerative applications.
The implications of this research extend beyond the laboratory. In the commercial sector, particularly in the energy sector, the development of such advanced biomaterials could open up new avenues for innovation. For instance, the enhanced mechanical properties of these hydrogels could be leveraged in the development of more durable and efficient energy storage devices, or in the creation of advanced materials for renewable energy applications.
Moreover, the ability to engineer tissues that can bear significant mechanical loads could have profound implications for the development of artificial organs and other medical devices. This could lead to a new era of personalized medicine, where tissues and organs are tailored to the specific needs of individual patients.
As we look to the future, the work of Gomes and her team serves as a testament to the power of interdisciplinary research. By bringing together expertise from chemistry, materials science, and biomedical engineering, they have made a significant stride towards addressing one of the most pressing challenges in regenerative medicine.
“The potential applications of these hydrogels are vast,” says Gomes. “From tissue engineering to energy storage, the possibilities are truly exciting.”
In the realm of biomedical materials, the journey towards creating the perfect hydrogel has been a long and winding one. But with the work of researchers like Maria Clara Gomes, we are one step closer to unlocking the full potential of these remarkable materials. As we continue to push the boundaries of what is possible, the future of regenerative medicine and beyond looks brighter than ever.