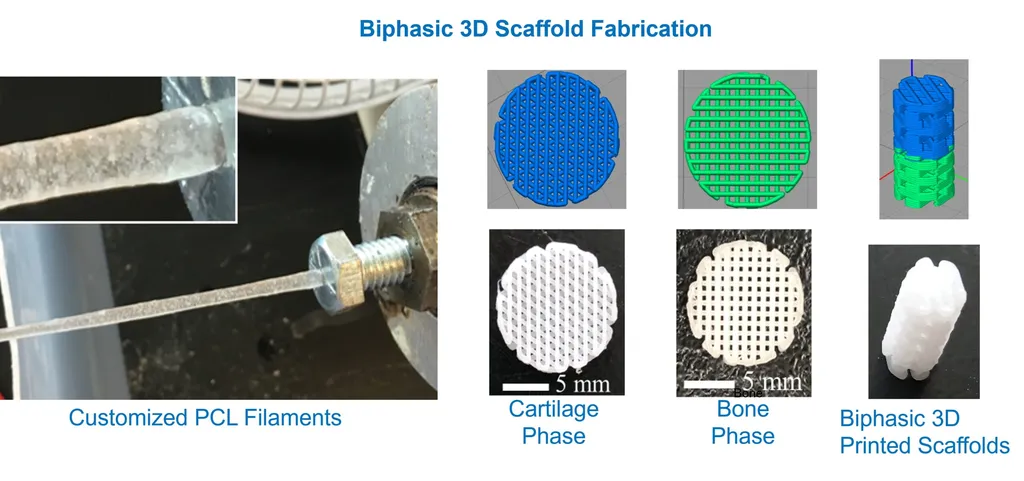
In a groundbreaking development that could revolutionize the treatment of osteochondral defects, researchers have successfully 3D-printed a bilayered scaffold designed to regenerate the subchondral plate. This innovative approach, detailed in a study published in *Materials Research Express* (which translates to *Materials Research Express* in English), addresses the complex challenges posed by subchondral plate degeneration, a condition that has long evaded effective treatment.
The study, led by Sena Su Torun from the Department of Bioengineering at Yildiz Technical University and the Center for Nanotechnology and Biomaterials Application and Research (NBUAM) at Marmara University, leverages Direct Ink Writing (DIW) technology to create scaffolds that mimic the intricate structure of native subchondral bone and calcified cartilage. “The cooperative existence of multiple layer types with different characteristics makes it difficult to meet the mechanical and biochemical requirements of subchondral tissue,” Torun explained. “Our goal was to develop a scaffold that could effectively replicate these properties.”
The bilayered scaffold is composed of poly(e-caprolactone), polyethylene glycol, and hydroxyapatite, a combination that enhances the scaffold’s swelling and degradation properties. The presence of hydroxyapatite, in particular, was found to significantly improve these characteristics, making the scaffold more conducive to tissue regeneration.
One of the most compelling aspects of this research is the mechanical characterization of the scaffold. The bone layer (BL) exhibited a Young’s modulus of 167.45 ± 57.51 MPa and a tensile strength of 4.05 ± 0.27 MPa, while the calcified cartilage layer (CCL) showed a lower modulus of 21.95 ± 0.43 MPa and a tensile strength of 2.33 ± 0.25 MPa. These values align closely with the mechanical gradient of native tissue, a critical factor for successful regeneration.
“The distinct tensile strength and stiffness gradients between the BL and CCL are crucial for mimicking the natural structure of the subchondral plate,” Torun noted. “This alignment is essential for the scaffold to function effectively in a biological environment.”
In vitro cell culture studies further demonstrated the scaffold’s biocompatibility, with cell viability exceeding 83% after seven days. This finding underscores the potential of the bilayered scaffold as a viable solution for treating subchondral plate degeneration.
The implications of this research extend beyond the medical field, with significant commercial impacts for the energy sector. The development of advanced materials and technologies for tissue engineering can drive innovation in other industries, including energy storage and conversion, where similar principles of material design and optimization are applied.
As the field of 3D printing continues to evolve, the successful development of this bilayered scaffold represents a significant step forward. It highlights the potential of additive manufacturing technologies to address complex biological challenges and paves the way for future advancements in tissue engineering and regenerative medicine.
In the words of Torun, “This research opens up new possibilities for the treatment of osteochondral defects and could have far-reaching implications for the energy sector and beyond.” With the publication of this study in *Materials Research Express*, the scientific community is one step closer to unlocking the full potential of 3D-printed scaffolds for medical and industrial applications.