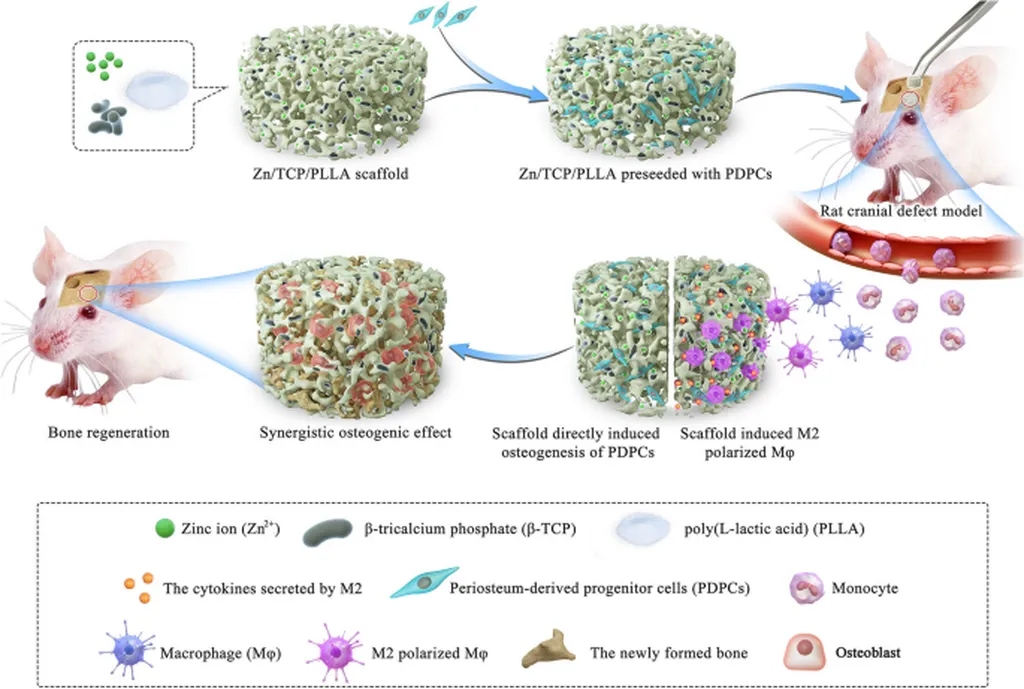
In the quest to overcome bone injury repair challenges, a groundbreaking study led by Yitong Liu from the Laboratory of Tissue Regeneration and Immunology at Capital Medical University has shed new light on the role of intracellular zinc ion transport in vascularized bone regeneration. Published in the journal *Bioactive Materials* (translated as *生物活性材料*), this research could potentially revolutionize stem cell therapy and biomaterial design, with significant implications for the medical and energy sectors.
Bone regeneration has long been a clinical hurdle, primarily due to local blood flow disorders and hypoxic microenvironments. Liu and their team established a mouse model of skull bone injury treated with bone marrow mesenchymal stem cells (BMSCs) to investigate this complex process. Their findings revealed that local BMSC transplantation stimulated vascularized bone regeneration, with matrix metalloproteinase (MMP)10 emerging as the key regulatory protein.
The study uncovered that the local hypoxic microenvironment induced mitochondrial permeability, leading to cytoplasmic zinc ion (Zn2+) accumulation. This accumulation was found to be crucial in activating the JAK1/STAT1/MMP-10 pathway. “We discovered that the inhibition of ZRT/IRT-like protein 6 (ZIP6) was the initiating factor in this process,” Liu explained. This insight into the importance of intracellular zinc ion homeostasis and ZIP proteins in transplanted MSC function opens new avenues for therapeutic strategies.
To capitalize on these findings, the researchers designed and engineered CD90@ZIF-8-ICG, a targeted delivery system that increases intracellular zinc ion content in local MSCs. This innovation regulates local MMP-10 production and angiogenesis during the early stages of healing. Indocyanine green (ICG) provided BMSCs with continuous photothermal stimulation in response to laser intervention, achieving stable improvement in bone-defect regeneration.
The implications of this research extend beyond medical applications. In the energy sector, understanding and manipulating stem cell behavior could lead to the development of advanced biomaterials for energy storage and conversion devices. For instance, the targeted delivery systems and photothermal stimulation techniques could inspire new approaches to designing more efficient and durable energy materials.
“This study not only elucidates the mechanisms of MSC therapy but also provides strategies for the design and development of stem-cell-based biomaterials,” Liu noted. The commercial potential of these advancements is substantial, with applications ranging from regenerative medicine to energy storage solutions.
As the scientific community continues to explore the intricacies of stem cell therapy and biomaterial design, this research serves as a beacon of innovation. By unraveling the regulatory importance of intracellular zinc ion homeostasis, Liu and their team have paved the way for future developments that could transform both medical and energy landscapes. The journey towards overcoming bone injury repair challenges has taken a significant step forward, thanks to the pioneering work published in *Bioactive Materials*.