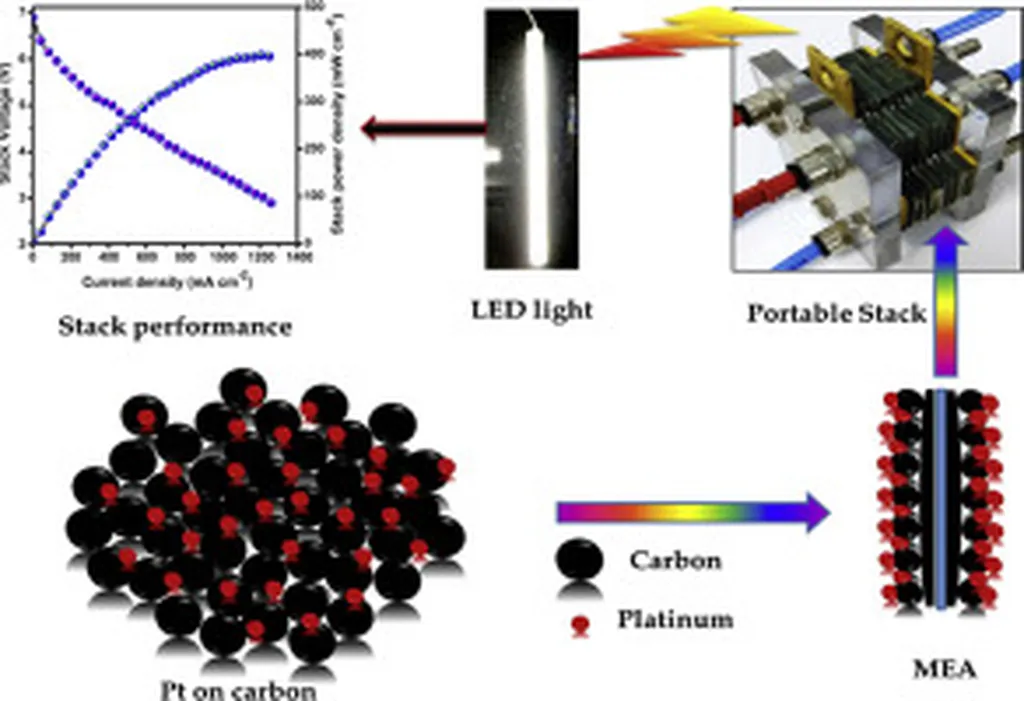
In the quest to make fuel cells more efficient and affordable, researchers have turned to an unlikely hero: carbon-encapsulated platinum subnanoclusters. A recent study led by Eoyoon Lee from the Department of Chemistry and Chemical Engineering at Inha University in South Korea has shed light on the remarkable potential of these tiny catalysts, offering a promising strategy to reduce platinum loadings while enhancing catalytic activity and stability.
The study, published in the journal *Applied Surface Science Advances* (translated as *Advances in Surface Science and Applied Technology*), focuses on the oxygen reduction reaction (ORR), a critical process in proton exchange membrane fuel cells. The ORR’s efficiency directly impacts the overall performance of these fuel cells, which are seen as a key technology for clean energy applications.
Lee and his team used spin-polarized density functional theory (DFT) calculations to develop and analyze carbon-encapsulated platinum and platinum-alloy subnanoclusters. Their findings revealed that these subnanoclusters offer a unique advantage: a facile four-electron ORR pathway via hydrogen peroxide decomposition with a low kinetic barrier. “The Pt6@C subnanocluster exhibited improved ORR activity with a higher onset potential of 0.60 V compared to the traditional Pt(111) catalyst,” Lee explained.
To further reduce platinum loadings and tune catalytic activity, the researchers introduced binary Pt3M3 alloy subnanoclusters, where M represents various metals from the 3d, 4d, and 5d blocks. Through screening these candidates using the *OOH adsorption energy as an activity descriptor, they identified new alloy subnanoclusters such as Pt3Co3, Pt3Rh3, Pt3Ta3, and Pt3Re3, which showed even better ORR activity relative to Pt6@C.
One of the key insights from the study is the role of the pz band center of the carbon sites. The effective charge transfer from the metal subnanocluster to the carbon shell leads to a down-shift of the pz band center. This, in turn, forms a bonding orbital between *OOH and carbon at a deeper energy level, strengthening *OOH adsorption and decreasing the overpotential.
The implications of this research are significant for the energy sector. By developing hybrid metal-carbon catalysts with highly reduced platinum loadings, the study paves the way for more efficient and cost-effective fuel cells. “Our findings provide valuable insights into the design of advanced catalysts for the ORR and other electrocatalysis applications,” Lee noted.
As the world continues to seek sustainable energy solutions, this research offers a compelling step forward. The ability to enhance catalytic activity while reducing the reliance on precious metals like platinum could revolutionize the commercial viability of fuel cells, making them a more attractive option for a wide range of applications. The study not only advances our understanding of subnanocluster catalysts but also opens new avenues for innovation in the field of electrocatalysis.