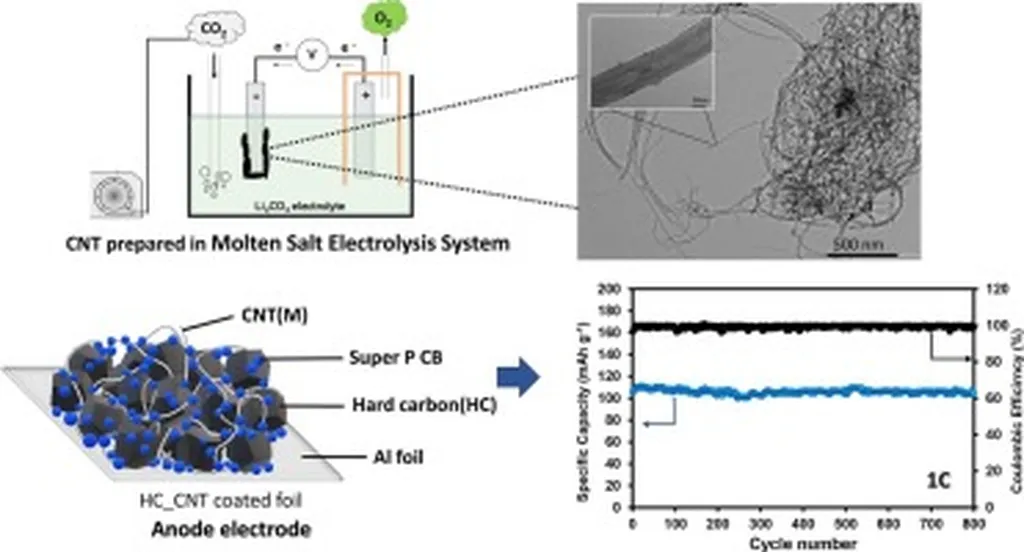
In the quest for sustainable and efficient energy solutions, scientists are turning to an unlikely ally: carbon dioxide. A recent review published in the journal *Applied Surface Science Advances* (which translates to *Advanced Studies in Applied Surface Science*) sheds light on a promising method for converting CO2 into valuable carbon nanotubes (CNTs) using molten salt electrolysis. This process not only offers a way to utilize greenhouse gases but also presents a potential energy-saving alternative to traditional synthesis methods.
At the helm of this research is I Ketut Rai Asmara Dipta, a chemist from the Department of Chemistry at Kookmin University in Seoul, South Korea. Dipta and his team have delved into the intricate world of electrode materials, exploring how different surfaces and properties influence the electrochemical synthesis of CNTs. “The key to unlocking the full potential of this method lies in understanding the interplay between the electrode materials and the CO2 conversion process,” Dipta explains. “By optimizing these interactions, we can enhance the efficiency and scalability of CNT production.”
Carbon nanotubes are renowned for their exceptional electrical, thermal, and mechanical properties, making them highly sought after for advanced technological applications. Traditional methods like chemical vapor deposition (CVD) have been the go-to for large-scale CNT production, but they come with significant energy costs. Molten salt electrolysis offers a greener alternative, simultaneously addressing CO2 utilization and energy savings.
The review critically analyzes various electrode materials, including metals, alloys, metal oxides, graphite, and liquid metals. Each material brings its unique advantages and challenges to the table. For instance, metals and alloys exhibit high catalytic activity, while graphite offers stability under oxygen evolution conditions. “The choice of electrode material is crucial,” Dipta notes. “It directly impacts the carbon–metal interactions and, consequently, the morphology and purity of the resulting CNTs.”
One of the major hurdles in this field is achieving high purity and structural control of the CNTs. Scaling up the process for industrial applications and enhancing energy efficiency are also pressing concerns. Dipta’s review aims to address these challenges by consolidating recent progress and identifying key knowledge gaps. “Our goal is to guide future innovation in the rational design of electrode materials,” he says. “This will pave the way for sustainable CNT production via electrochemical CO2 conversion.”
The implications of this research extend beyond the laboratory. In the energy sector, the ability to convert CO2 into valuable nanomaterials could revolutionize carbon capture and utilization technologies. By integrating these methods into industrial processes, companies can reduce their carbon footprint while producing high-value materials. “This is a win-win scenario,” Dipta emphasizes. “We can mitigate climate change and create economic value simultaneously.”
As the world grapples with the challenges of climate change and energy sustainability, research like Dipta’s offers a glimmer of hope. By harnessing the power of electrochemical processes and innovative materials, we can turn a greenhouse gas into a valuable resource. The journey towards sustainable energy is fraught with challenges, but with each breakthrough, we inch closer to a cleaner, greener future.