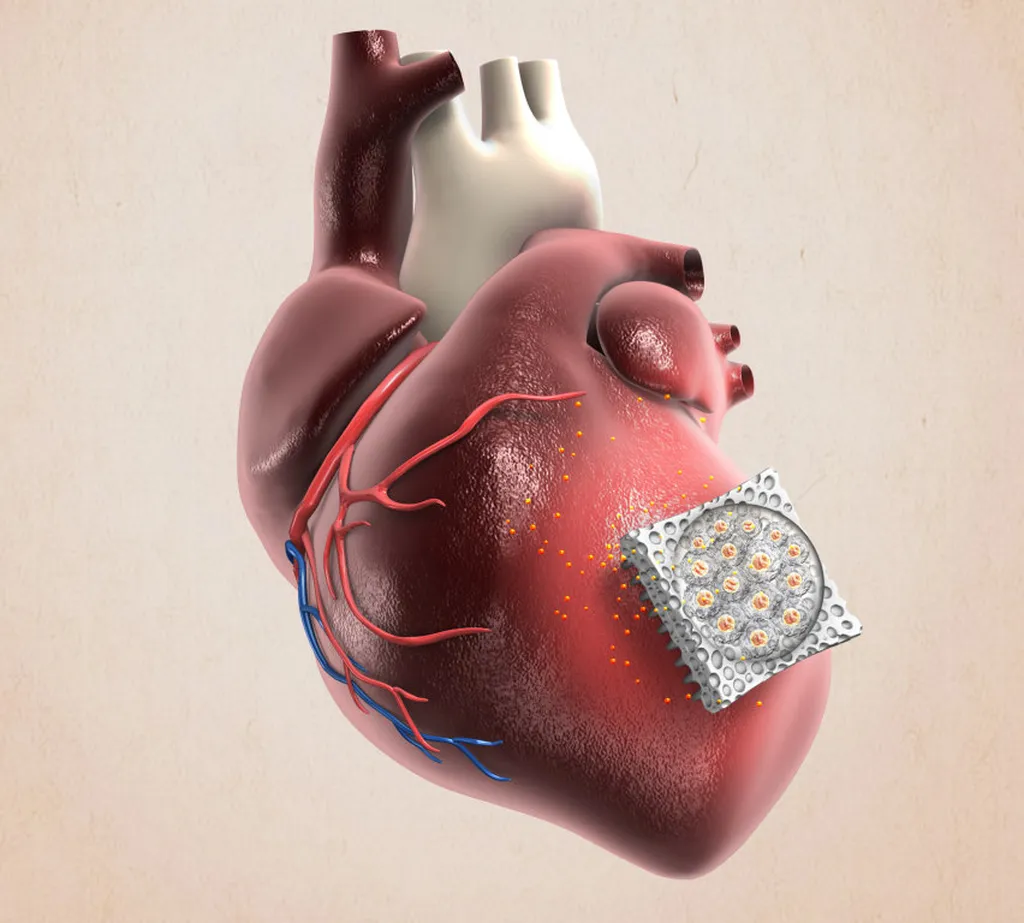
In the relentless pursuit of innovative cardiac therapies, a team of researchers led by Jianqiu Yang from the Department of Cardiothoracic Surgery at Nanchang University has developed a groundbreaking intelligent cardiac patch, SMM@Gel, that shows remarkable promise in treating myocardial infarction (MI). This advanced biomaterial, detailed in a recent study published in *Bioactive Materials* (translated as *活性材料*), combines reactive oxygen species (ROS)-responsive hydrogel with targeted drug delivery to improve heart function post-infarction.
Myocardial infarction, commonly known as a heart attack, triggers a cascade of detrimental events, including inflammation, fibrosis, and ventricular remodeling, often leading to heart failure. Traditional treatments have struggled to address these complex processes effectively. However, the SMM@Gel patch offers a dual-action approach: it scavenges harmful ROS and promotes anti-inflammatory and angiogenic effects, potentially revolutionizing MI treatment.
The SMM@Gel patch is composed of a PVA-TSPBA hydrogel matrix reinforced through solvent exchange and salting-out technology. This matrix is loaded with mannose-functionalized, danshensu sodium-loaded hollow mesoporous polydopamine nanoparticles (Sa@mPDA-Man). The intelligent design ensures sustained drug release and effective ROS scavenging, addressing key challenges in cardiac repair.
In vitro studies demonstrated that SMM@Gel significantly promoted endothelial tube formation and M2 macrophage polarization, reducing inflammation. “The patch’s ability to target macrophages and modulate their behavior is a game-changer,” said Yang. “It allows us to create a more favorable microenvironment for cardiac repair.”
In vivo experiments in MI rats revealed even more promising results. The SMM@Gel patch preserved left ventricular ejection fraction (LVEF), normalized ventricular dimensions, and enhanced wall thickness. Histological analysis showed reduced infarct size, decreased inflammation, and improved neovascularization. RNA sequencing identified pathways linked to angiogenesis, inflammation resolution, and extracellular matrix remodeling, underscoring the patch’s multifaceted therapeutic potential.
The implications of this research extend beyond the laboratory. As Yang noted, “This technology has the potential to transform how we treat myocardial infarction, offering a more comprehensive and effective approach to cardiac repair.” The commercial impact for the medical sector could be substantial, with the development of advanced biomaterials that combine drug delivery and ROS scavenging opening new avenues for treating heart disease.
The study’s findings highlight the potential of SMM@Gel as a dual-action therapy for MI, combining ROS scavenging, anti-inflammatory effects, and angiogenic promotion to mitigate post-MI remodeling and preserve cardiac function. As research progresses, this innovative approach could pave the way for more effective treatments, ultimately improving patient outcomes and reshaping the future of cardiac care.