In the heart of Guangzhou, China, a team of researchers led by Dr. Qiuming Pan at the Department of Neurosurgery, Nanfang Hospital, Southern Medical University, is unraveling the intricate dance between cellular senescence and cancer. Their work, recently published in the journal *Biomedical Engineering and Materials* (BMEMat), is shedding new light on how understanding and manipulating this cellular phenomenon could revolutionize cancer treatment.
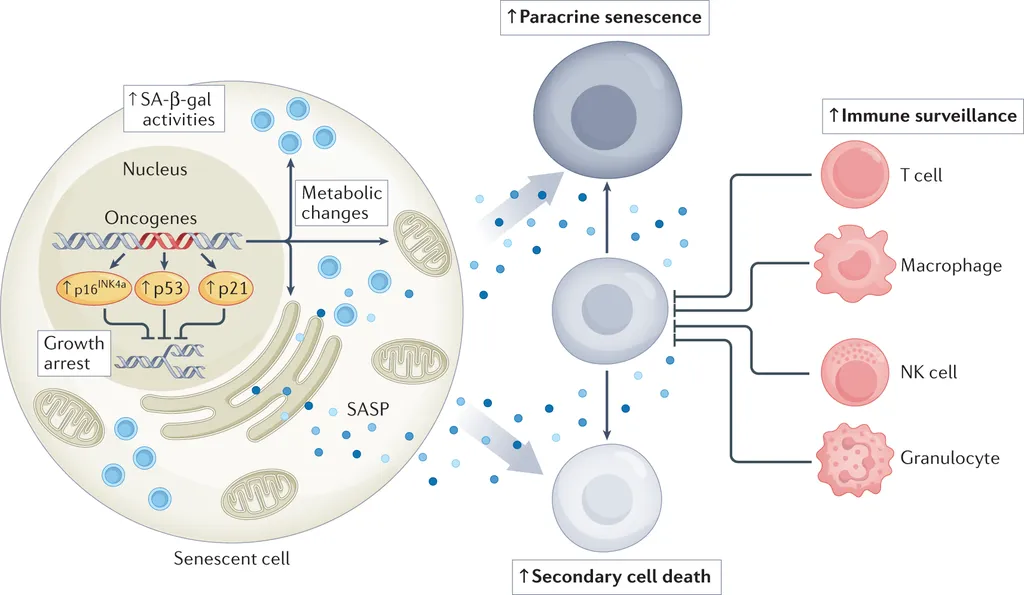
Cellular senescence, a state where cells permanently stop dividing, has long been a double-edged sword in cancer. On one hand, it can halt the proliferation of cancer cells, acting as a natural brake on tumor growth. On the other, senescent cells can secrete a mix of inflammatory factors and cytokines, collectively known as the senescence-associated secretory phenotype (SASP), which can sometimes fuel cancer progression.
Dr. Pan and his team have been delving into the mechanisms behind this paradox. “We’ve known for some time that cellular senescence can both suppress and promote cancer,” Dr. Pan explains. “What we’re trying to do is understand the context and the mechanisms that determine which of these outcomes prevails.”
Their review article provides a comprehensive overview of the major triggers of senescence and the regulatory mechanisms at play. Importantly, they also explore the dual role of SASP in cancer, highlighting its potential as a therapeutic target.
One of the most promising avenues they discuss is the “One-Two punch” sequential treatment approach. This strategy involves first inducing senescence in cancer cells, then using a second treatment to eliminate the senescent cells and their pro-inflammatory secretions. Early studies suggest this approach could be more effective than current treatments, but challenges remain.
The implications of this research extend beyond the lab. As we understand more about the role of cellular senescence in cancer, we open up new avenues for drug development. Companies in the pharmaceutical and biotechnology sectors are already taking notice, with several startups focusing on senolytic drugs—compounds that selectively eliminate senescent cells.
Moreover, this research could have broader implications for the energy sector. As Dr. Pan points out, “Understanding the metabolic changes that occur during senescence could help us develop more efficient and sustainable energy solutions.” For instance, the metabolic shifts in senescent cells could inspire new approaches to bioenergy production or waste management.
However, the path forward is not without hurdles. As the authors note in their article, we still have much to learn about the complex interplay between senescence, SASP, and cancer. But with each discovery, we inch closer to harnessing the power of cellular senescence for good.
In the words of Dr. Pan, “This is a complex puzzle, but every piece we put in place brings us one step closer to a cure.” And with each step, we edge closer to a future where cancer is not just managed, but conquered.