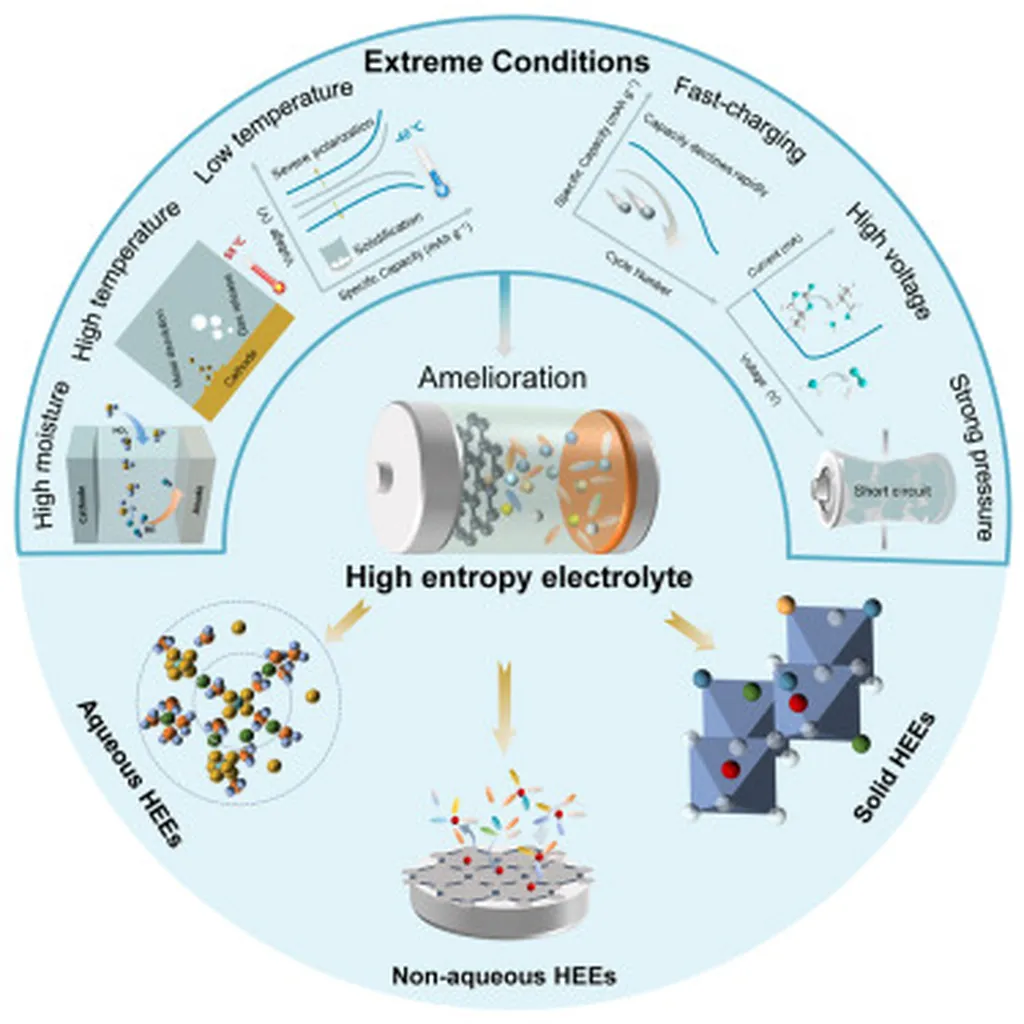
In the relentless pursuit of efficient energy storage solutions, researchers are turning to an unconventional ally: entropy. A recent study published in *Materials Reports: Energy* (translated from Chinese as *Energy Materials Reports*) explores the potential of high-entropy liquid electrolytes to revolutionize rechargeable batteries, offering a glimpse into a future where batteries operate more efficiently and reliably across a broader range of temperatures.
At the heart of this research is the concept of entropy, a thermodynamic measure of disorder. Traditionally associated with high-entropy alloys, this principle is now being applied to liquid electrolytes, both aqueous and non-aqueous, to enhance their performance. “High-entropy materials leverage the synergistic effects of multiple components to achieve superior properties,” explains lead author Mingcong Tang, an assistant professor in the Department of Mechanical Engineering at The Hong Kong Polytechnic University. “By manipulating entropy, we can significantly improve the ionic conductivity and stability of electrolytes, which are critical for battery performance.”
The study highlights the unique advantages of high-entropy liquid electrolytes, including their ability to operate effectively in extreme temperatures. This is a game-changer for industries that rely on energy storage solutions in harsh environments, such as renewable energy systems and electric vehicles. “The potential to widen the operational temperature range of batteries is a significant step forward,” says Tang. “It addresses a key challenge in the energy sector, where batteries often struggle to perform optimally in extreme conditions.”
However, the path to commercialization is not without its hurdles. The complex compositions of high-entropy electrolytes mean that even minor changes in formulation can lead to substantial variations in performance. This complexity presents both a challenge and an opportunity for researchers and industry professionals alike. “Understanding and controlling these variations is crucial for the practical implementation of high-entropy electrolytes,” Tang notes. “It requires a deep dive into the interplay between entropy and electrochemical behavior, which is what we aim to achieve through our research.”
The study not only introduces the fundamentals of entropy tuning but also surveys recent advances in high-entropy liquid electrolytes. It provides a comprehensive analysis of the interplay between entropy and electrochemical behavior, offering valuable insights for future research and development. “Our goal is to pave the way for the practical implementation of high-entropy liquid electrolytes in next-generation energy storage systems,” Tang explains. “This involves developing design strategies that can harness the full potential of these materials.”
The implications of this research extend far beyond the laboratory. As the world grapples with the growing severity of environmental challenges, the need for efficient energy storage solutions has never been more pressing. High-entropy liquid electrolytes offer a promising avenue for advancing renewable energy technologies, potentially reshaping the energy sector and accelerating the transition to a more sustainable future.
In the words of Mingcong Tang, “The journey towards practical implementation is just beginning, but the potential is immense. By pushing the boundaries of entropy manipulation, we are opening new doors to innovation in energy storage.” As researchers continue to unravel the complexities of high-entropy materials, the energy sector stands on the brink of a transformative era, where batteries are not just more efficient but also more adaptable to the demands of a changing world.