In a groundbreaking development that could reshape the energy and chemical industries, researchers have unveiled a novel approach to urea electrosynthesis, offering a more efficient and selective method for converting carbon dioxide (CO2) and nitrate (NO3−) into urea. This innovation, published in the journal *InfoMat* (translated as *Information Materials*), holds significant promise for advancing sustainable chemical manufacturing and energy storage solutions.
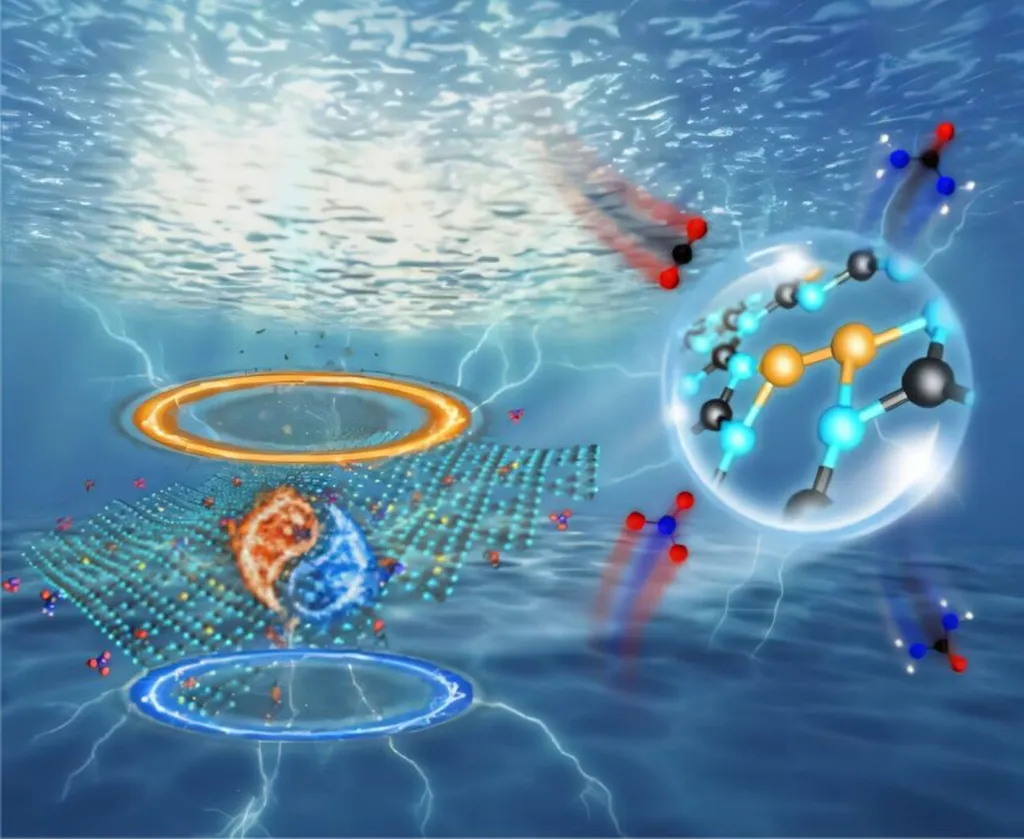
At the heart of this research is the dynamic interplay between vicinal Fe/Cu diatoms and alloy-like Fe–Cu sites, a discovery led by Su Wang from Guizhou University in China. Wang and his team have demonstrated that this reversible evolution enables a cascade protonation process, significantly enhancing the efficiency and selectivity of urea production. “The key to our success lies in the dynamic evolution of these active sites, which allows us to overcome the traditional constraints of adsorption scaling and spatial limitations,” Wang explained.
The implications for the energy sector are profound. Urea, a vital chemical in agriculture and industry, is traditionally produced through energy-intensive processes. The new method not only boosts the yield of urea but also does so with remarkable efficiency. The researchers achieved an ultrahigh urea yield of 2421.2 μg h−1 mg−1, a Faraday efficiency (FE) of 70.4%, and a C-selectivity of 96.7%. These figures surpass the performance of state-of-the-art dual-site electrocatalysts, marking a significant leap forward in the field.
The dynamic evolution of active sites, as revealed by operando spectroscopy and theoretical calculations, facilitates the selective adsorption and hydrogenation of NO3− and CO2 into key intermediates (*NO and *CO). The alloy-like Fe–Cu sites, formed in situ due to declined metal surface free energy driven by electron transfer, further enhance C–N coupling and subsequent protonation to selectively produce urea. This process then dynamically reverts to vicinal Fe/Cu diatoms, creating a continuous cycle of efficient catalysis.
“This research provides new insights into the relay catalytic strategy for urea electrosynthesis by modulating the dynamic atomic-scale evolution of active sites,” Wang noted. The findings could pave the way for more sustainable and cost-effective chemical production methods, reducing the environmental impact of traditional industrial processes.
As the world seeks innovative solutions to combat climate change and transition to a greener economy, advancements like this are crucial. The ability to efficiently convert CO2 into valuable chemicals not only mitigates greenhouse gas emissions but also opens up new avenues for energy storage and utilization. The research by Wang and his team represents a significant step forward in this endeavor, offering a glimpse into the future of sustainable chemical manufacturing.
With the publication of this work in *InfoMat*, the scientific community now has a robust framework to build upon, potentially leading to further breakthroughs in electrosynthesis and related fields. The dynamic evolution of active sites, as demonstrated in this study, could inspire new approaches to catalysis, driving innovation across various industries. As researchers continue to explore and refine these methods, the potential for commercial impact and environmental benefits grows ever more promising.