In the relentless pursuit of enhancing the durability and longevity of steel structures, particularly in harsh industrial environments, a team of researchers led by WANG Fengtao from the School of Materials Science and Engineering at Hunan University of Technology has made significant strides. Their work, published in the journal *Cailiao Baohu* (which translates to *Materials Protection*), focuses on the corrosion resistance of Q420qNH weathering steel under industrial atmospheric exposure, offering promising insights for the energy sector and beyond.
Weathering steel, known for its ability to form a protective rust layer that inhibits further corrosion, is widely used in infrastructure projects, including bridges, buildings, and industrial facilities. However, its performance can vary significantly depending on environmental conditions. The research team aimed to evaluate the effects of different stabilizing agents on the corrosion resistance of this steel, providing practical guidance for its application in industrial atmospheres.
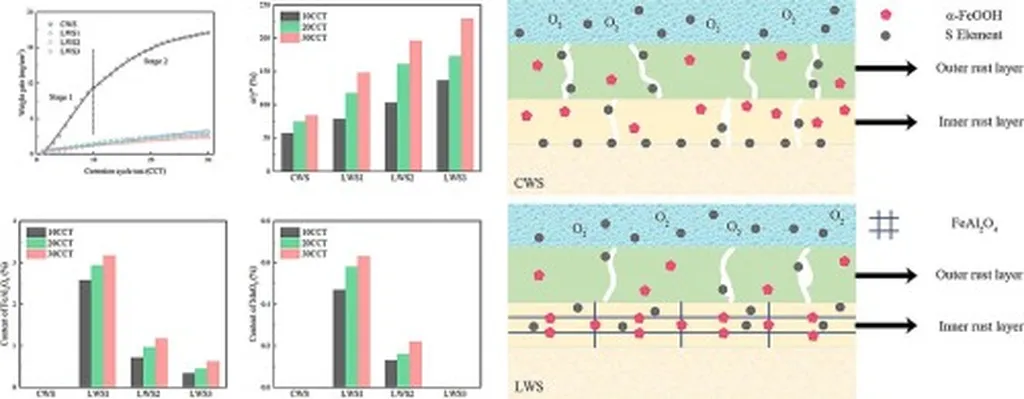
The study involved two groups of stabilizing agents, labeled Group C and Group F, which were applied to Q420qNH steel specimens. These specimens underwent an 8-cycle stabilization treatment in the laboratory, followed by a 3-month exposure test in an industrial atmosphere. The researchers employed various analytical techniques, including corrosion kinetics, X-ray diffraction (XRD), scanning electron microscopy (SEM), and electrochemical analysis, to compare the corrosion resistance of treated and untreated specimens.
The results were compelling. “Under industrial atmosphere exposure, the stabilized specimens exhibited a higher initial corrosion rate, which decreased in later stages,” noted WANG Fengtao. This initial increase in corrosion rate is a crucial observation, as it indicates that the stabilizing agents accelerate the formation of a protective rust layer. Over time, this layer becomes denser and more compact, significantly enhancing the steel’s resistance to further corrosion.
The rust layers of both treated and untreated specimens were primarily composed of α-FeOOH, γ-FeOOH, β-FeOOH, and Fe3O4/γ-Fe2O3 phases. However, the stabilization treatment led to an increase in the content of α-FeOOH, with Group C achieving the highest content at 53%. This phase is known for its protective properties, contributing to the improved corrosion resistance observed in the treated specimens.
Among all the specimens, those treated with Group C stabilizing agent exhibited the densest rust layer, the highest self-corrosion potential, and the strongest corrosion resistance. Group F also performed well, but the bare steel fared the worst. The stabilizing agents effectively shortened the stabilization period of the rust layer, with Group C demonstrating the best effect on promoting the formation of a stable rust layer.
The implications of this research are significant for the energy sector, where steel structures are often exposed to harsh industrial atmospheres. By enhancing the corrosion resistance of weathering steel, these stabilizing treatments can extend the lifespan of critical infrastructure, reduce maintenance costs, and improve overall safety. As WANG Fengtao explained, “The stabilizing agents not only improve the corrosion resistance but also accelerate the formation of a protective rust layer, which is crucial for the long-term performance of steel structures in industrial environments.”
This study, published in *Cailiao Baohu*, provides a solid foundation for further research and practical applications. As the energy sector continues to demand more durable and reliable materials, the insights gained from this research could shape future developments in the field, ensuring that steel structures remain robust and resilient in the face of challenging environmental conditions.