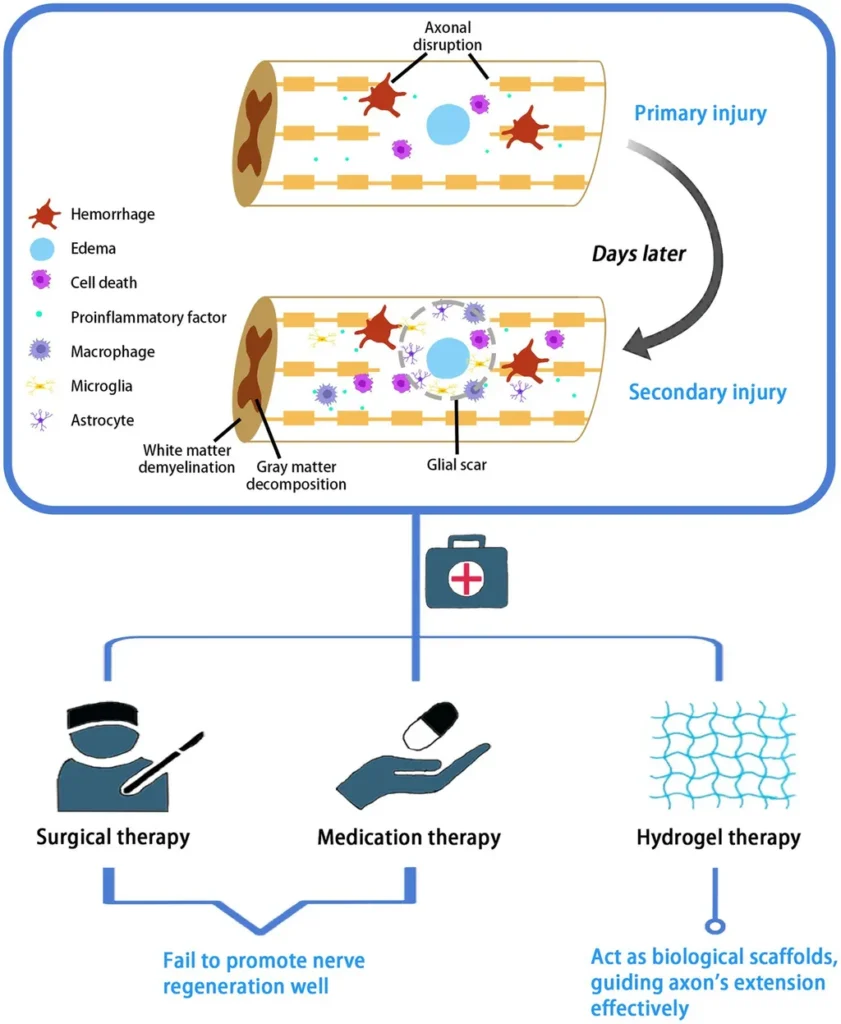
In a groundbreaking development that could revolutionize the treatment of spinal cord injuries (SCI), researchers have engineered a multifunctional hydrogel that not only promotes neural regeneration but also enhances the functional maturation of newly formed neurons. The study, led by Yiqian Luo from the Department of Spine Surgery at The Third Affiliated Hospital of Sun Yat-Sen University in Guangzhou, China, was recently published in the journal *Bioactive Materials* (translated to English as *活性材料*).
The innovative hydrogel, named Poly(LA-Cho)/SS31 (PLCS), is synthesized in a single step using lipoic acid (LA), choline bicarbonate, and elamipretide (SS31). This unique formulation offers a range of functionalities, including injectability, self-healing ability, tissue adhesion, and sequential drug release. “The one-step synthesis method without catalysts and organic solvents is a significant advancement,” Luo explains. “It allows us to integrate physical and biological functions of the hydrogel through simple mixing, making it highly promising for clinical translation.”
The PLCS hydrogel works by first releasing SS31 to scavenge mitochondrial reactive oxygen species (ROS) and alleviate mitochondrial dysfunction. Following this, LA is continuously released to further scavenge ROS. This dual-action approach not only promotes the differentiation of Neural Stem Cells (NSCs) into cholinergic neurons but also increases acetyl-CoA levels and supplies choline, essential substrates for acetylcholine synthesis in the newly formed neurons. “This sequential release mechanism ensures that the hydrogel provides sustained support for neural regeneration and functional maturation,” Luo adds.
In rat models of SCI, the PLCS hydrogel demonstrated robust nerve regeneration and significantly improved motor, sensory, and bladder functions. RNA sequencing suggested that the PI3K-Akt pathway may contribute to spinal cord repair, opening new avenues for further research and potential therapeutic interventions.
The implications of this research extend beyond the immediate medical applications. The development of such advanced biomaterials could pave the way for innovative treatments in the energy sector, particularly in areas requiring precise and controlled drug delivery systems. The hydrogel’s ability to integrate multiple functionalities in a single, easily synthesizable form could inspire new approaches to energy storage, release, and management.
As the field of biomaterials continues to evolve, the work of Luo and his team represents a significant step forward in the quest for effective SCI treatments. The simplicity and efficiency of the one-step synthesis method, combined with the hydrogel’s multifunctional capabilities, offer a highly promising strategy for clinical translation. This research not only advances our understanding of neural regeneration but also sets the stage for future developments in both medical and energy sectors.