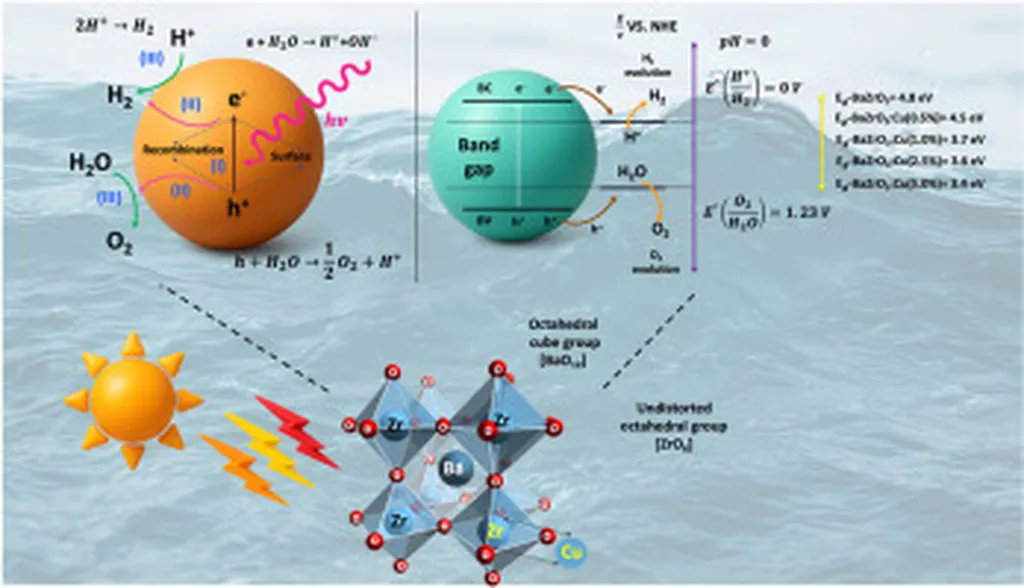
In the pursuit of sustainable energy solutions, scientists are continually exploring innovative materials to enhance the efficiency of water splitting, a process that could revolutionize hydrogen production. A recent study published in *SusMat* (Sustainable Materials) has shed light on a promising breakthrough in this arena, with implications that could resonate throughout the energy sector.
Researchers, led by Prathamesh Chougale from the Department of Chemistry at Jaysingpur College in Maharashtra, India, have uncovered the potential of copper-doped cobalt ferrite (CuCoFe2O4) as a highly effective bifunctional electrocatalyst. The study, which delves into the role of oxygen vacancies in catalytic activity, offers a fresh perspective on optimizing the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) for water splitting.
The team synthesized a series of Cu-doped mixed spinel cobalt ferrites, meticulously investigating their electrochemical performance. Their findings revealed that Cu doping significantly enhances charge transfer and promotes the formation of oxygen vacancies, both of which are crucial for improving reaction kinetics. Among the synthesized materials, CuCoFe0.5 emerged as the standout performer, demonstrating the lowest overpotential for both OER (280 mV) and HER (−143 mV), as well as a remarkable cell voltage of 1.66 V during 20 hours of continuous water splitting.
“This enhanced performance can be attributed to the increased electrochemically active surface area (ECSA) and the abundance of oxygen vacancies, which serve as active sites for both HER and OER,” explained Chougale. The study’s results underscore the critical role of oxygen vacancies in boosting catalytic efficiency, paving the way for the design of next-generation spinel ferrite-based electrocatalysts.
One of the most intriguing aspects of this research is the application of Long Short-Term Memory (LSTM) memory cells for forecasting electrochemical performance. By leveraging this advanced machine learning technique, the researchers were able to predict performance improvements with a 30% accuracy, highlighting the potential of AI in accelerating materials science discoveries.
The implications of this research for the energy sector are substantial. As the world shifts towards cleaner energy sources, the demand for efficient and cost-effective hydrogen production methods is on the rise. The development of highly active and stable bifunctional electrocatalysts, such as CuCoFe2O4, could significantly enhance the viability of water splitting technologies, bringing us one step closer to a sustainable energy future.
Moreover, the integration of AI-driven forecasting in materials science opens up new avenues for rapid innovation. As Chougale noted, “The use of LSTM memory cells not only provides valuable insights into the electrochemical performance of our materials but also offers a powerful tool for guiding future research and development efforts.”
In conclusion, this groundbreaking study not only advances our understanding of the role of oxygen vacancies in catalytic activity but also showcases the transformative potential of AI in the field of materials science. As the energy sector continues to evolve, the insights gained from this research could prove instrumental in shaping the future of sustainable energy production.