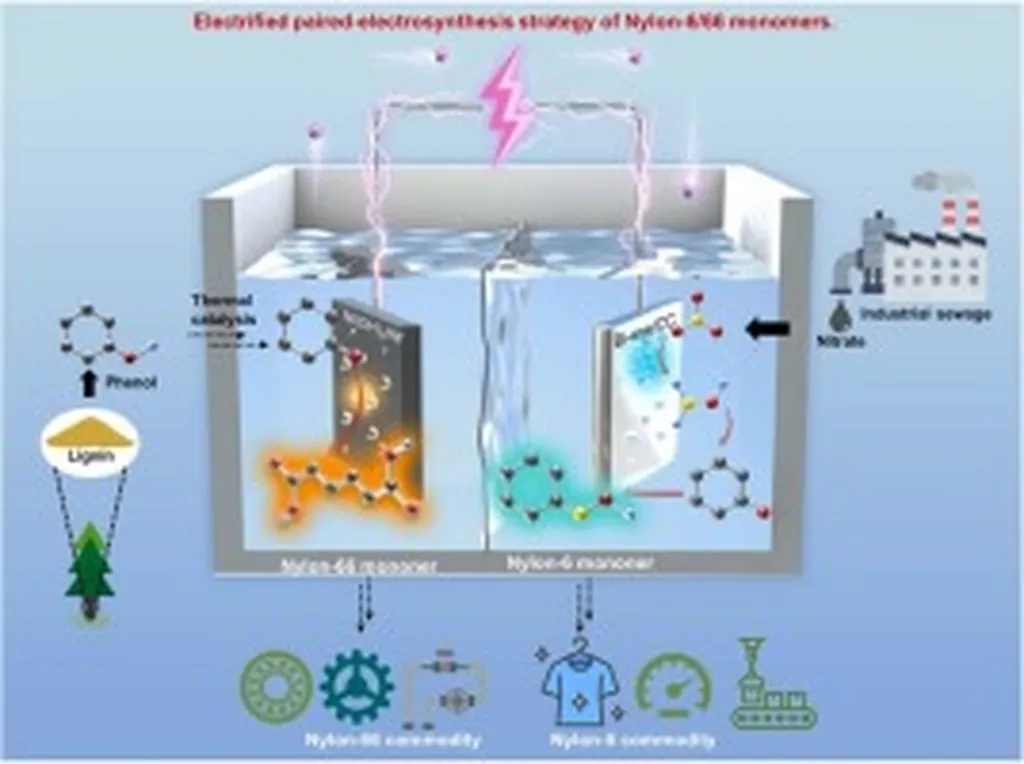
In a significant stride towards sustainable chemical manufacturing, researchers have developed a novel electrocatalyst that could revolutionize the production of Nylon monomers. The study, led by Chia-Hui Yen from the Department of Chemical Engineering at National Cheng Kung University in Taiwan, presents a green alternative to traditional methods, with substantial implications for the energy sector.
The research, published in the journal ‘Applied Surface Science Advances’ (or ‘Advances in Applied Surface Science’ in English), focuses on the electrocatalytic reduction of adiponitrile (e-ADNRR), a crucial process for synthesizing Nylon 6 and Nylon 6,6 monomers. Traditional methods rely on thermocatalytic hydrogenation, which is energy-intensive and less environmentally friendly. The new approach uses water as a proton source, making it a more sustainable option.
At the heart of this innovation is a nickel submicron-particulate film-modified electrode, dubbed Ti|microNi. This electrode, containing defective nickel oxide (NiOx) and metallic nickel, exhibits high performance under environmentally benign conditions. “The Ni2+ centers in the defective NiOx are low-coordinated and have a unique pyramidal symmetry with an oxygen vacancy,” explains Yen. This structural feature is key to the electrode’s efficiency.
The study systematically investigated various electrode preparation and electrosynthetic conditions, optimizing factors like electrolyte pH, adiponitrile concentration, and the concentration of quaternary alkyl ammonium salt. The results were impressive: under optimal conditions, the Ti|microNi electrode achieved an overall product current efficiency of approximately 98.5% at industrially relevant current, and near-neutral pH, without the need for organic cosolvents.
The commercial impacts of this research are substantial. By eliminating the use of organic cosolvents, the process not only reduces the risk of adiponitrile decomposition but also cuts down on energy consumption. This could lead to more cost-effective and eco-friendly production of Nylon monomers, a significant boon for the energy sector and the chemical industry at large.
Moreover, the study presents a novel strategy for designing high-performance electrocatalysts, paving the way for future developments in organic electrosynthesis. As Yen notes, “This research opens up new possibilities for efficient and sustainable chemical manufacturing processes.”
The findings of this study could indeed shape the future of the field, offering a glimpse into a more sustainable and energy-efficient industrial landscape. With further research and development, the Ti|microNi electrode and similar innovations could become a cornerstone of green chemical manufacturing, driving the energy sector towards a more sustainable future.