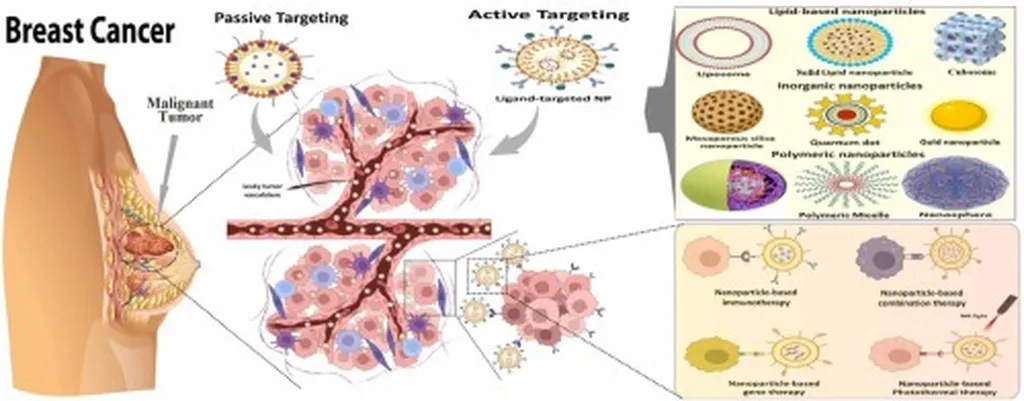
In the relentless pursuit of effective cancer treatments, researchers have often turned to nature’s vast chemical library for inspiration. A recent study published in *Macromolecular Materials and Engineering* (which translates to “Macromolecular Materials and Engineering” in English) has encapsulated a promising plant-derived compound within a sophisticated nanoparticle delivery system, potentially revolutionizing breast cancer treatment. The lead author, Preeyanuch Manohong from the Department of Biochemistry at Mahidol University in Bangkok, Thailand, and her team have developed a targeted approach that could minimize side effects and enhance therapeutic efficacy.
Mallotumide A (MA), a novel cycloheptapeptide isolated from the roots of Mallotus spodocarpus, has shown remarkable anticancer activity, particularly against triple-negative breast cancer. However, its poor water solubility and high toxicity to both cancer and normal cells have limited its clinical potential. To overcome these challenges, Manohong and her team encapsulated MA within poly(lactic-co-glycolic acid) (PLGA) nanoparticles and coated them with riboflavin-modified chitosan, creating a targeted delivery system.
“The encapsulation of MA within PLGA nanoparticles and the subsequent coating with riboflavin-modified chitosan significantly enhanced the compound’s cellular uptake in cancer cells while reducing its toxicity in normal cells,” Manohong explained. This targeted approach not only improves the therapeutic index of MA but also opens new avenues for the delivery of other hydrophobic and toxic compounds.
The study demonstrated that the MA-loaded PLGA/chitosan nanoparticles were spherical, with an average size of 300 nanometers and a zeta potential of +11.96 millivolts. The nanoparticles exhibited enhanced cellular uptake in both breast cancer cell lines (MDA-MB-231 and MCF-7) in a dose- and time-dependent manner. Importantly, the nanoparticles significantly inhibited the viability, migration, and invasion of MDA-MB-231 cells, which are known for their aggressive nature and poor prognosis.
The implications of this research extend beyond the immediate applications in breast cancer treatment. The development of targeted nanoparticle delivery systems could have profound impacts on the pharmaceutical and biotechnology industries, enabling the development of more effective and safer therapies for a wide range of diseases. As Manohong noted, “This study not only advances our understanding of targeted drug delivery but also paves the way for the development of novel therapeutic strategies that can overcome the limitations of conventional treatments.”
In the broader context, the encapsulation of plant-derived compounds within advanced nanoparticle systems represents a convergence of traditional medicine and cutting-edge technology. This synergy could lead to the discovery of new therapeutic agents and the development of innovative delivery systems that can address some of the most pressing challenges in healthcare.
As the field of nanomedicine continues to evolve, the work of Manohong and her team serves as a testament to the potential of interdisciplinary research. By combining insights from biochemistry, materials science, and cancer biology, they have developed a targeted delivery system that could transform the way we approach cancer treatment. The study, published in *Macromolecular Materials and Engineering*, highlights the importance of continued investment in basic and applied research, as well as the need for collaboration across scientific disciplines.
In the quest for more effective and targeted cancer therapies, the encapsulation of Mallotumide A within PLGA/chitosan nanoparticles represents a significant step forward. As the research community continues to explore the potential of plant-derived compounds and advanced delivery systems, the possibilities for improving patient outcomes and revolutionizing cancer treatment are vast and promising.