In the quest for safer and more cost-effective energy storage solutions, researchers have turned their attention to rechargeable magnesium batteries (RMBs). These batteries promise enhanced safety and reduced costs compared to their lithium-ion counterparts, but conventional electrolytes have posed significant challenges. A recent study published in the *Review of Materials Research* (translated from Chinese as “Materials Research Review”) sheds light on the potential of boron-based electrolytes, offering a promising path forward for the energy sector.
The study, led by Kangjie Xu from the State Key Laboratory of Materials-Oriented Chemical Engineering at Nanjing Tech University, focuses on the design and optimization of boron-based electrolytes for RMBs. Traditional chlorine-containing electrolytes have been hindered by their corrosive nature and low oxidative stability. In contrast, boron-based electrolytes offer superior stability and structural tunability, making them an attractive alternative.
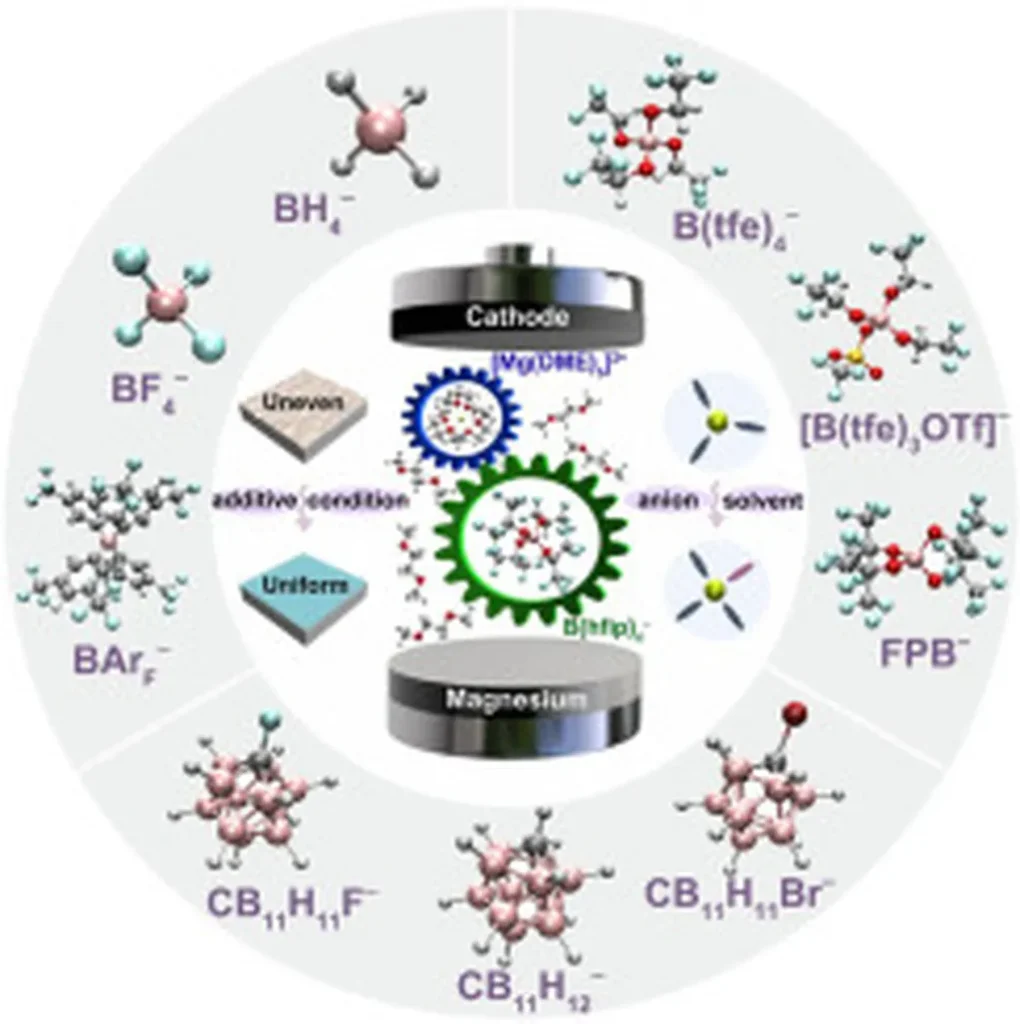
Xu and his team highlight three main classes of boron-based electrolytes: borohydride-based, carborane-based, and borate-based systems. Among these, the borate-based electrolyte using the Mg[B(hfip)4]2 salt stands out as a cost-performance benchmark. This compound achieves a favorable balance between electrochemical performance and synthetic scalability, making it a strong candidate for commercial applications.
The researchers propose two key approaches to further enhance the electrochemical performance of Mg[B(hfip)4]2: the incorporation of functional additives and the regulation of solvation structure. “By fine-tuning the solvation structure and adding specific additives, we can significantly improve the electrolyte’s performance,” Xu explains. Theoretical simulations play a pivotal role in understanding these mechanisms, providing valuable insights into solvation and interfacial dynamics.
The study also critically assesses the industrial application prospects of this electrolyte system. While the potential is substantial, further research is needed to optimize these electrolytes for real-world use. Xu emphasizes the importance of continued innovation in this field, stating, “Our work provides a roadmap for the advancement of RMB technologies, but there is still much to explore.”
The implications of this research extend beyond the laboratory. As the energy sector seeks to transition to more sustainable and efficient storage solutions, the development of high-performance electrolytes could be a game-changer. Boron-based electrolytes, with their superior stability and tunability, offer a promising avenue for achieving these goals.
This study not only advances our understanding of boron-based electrolytes but also paves the way for future developments in energy storage technology. As researchers continue to refine these electrolytes, the potential for widespread commercial impact grows ever closer. The journey towards safer, more cost-effective energy storage solutions is well underway, and boron-based electrolytes are at the forefront of this exciting frontier.