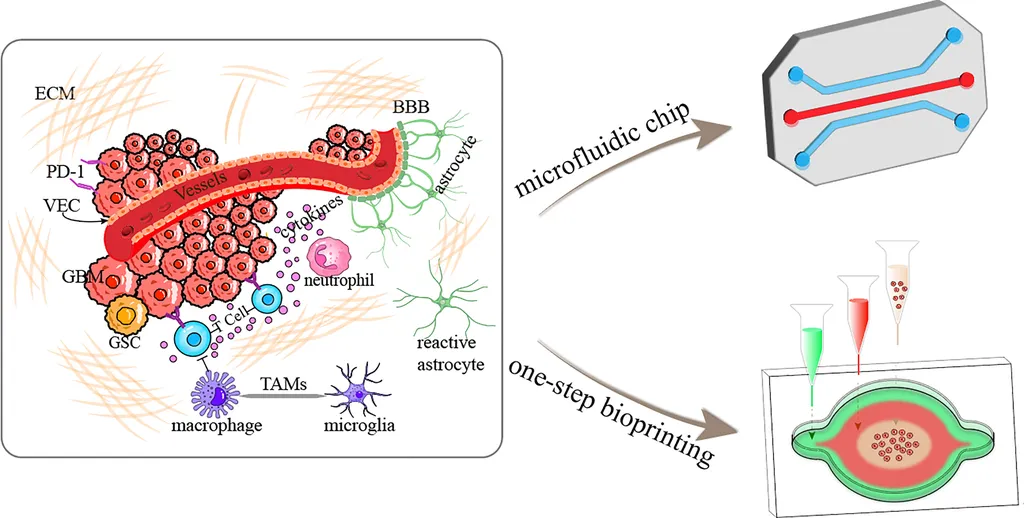
In the relentless battle against glioma, a highly aggressive brain tumor, researchers are turning to an innovative technology that could revolutionize our understanding and treatment of this complex disease. Organ-on-a-chip technology, specifically glioma-on-a-chip (GoC) models, is emerging as a powerful tool to bridge the gap between preclinical research and clinical outcomes. This cutting-edge approach, detailed in a recent review published in ‘Kleine Wissenschaft’ (Small Science), offers a promising path towards more personalized and effective therapies.
At the forefront of this research is Su Liu, a scientist at the Nanomaterials in Health Laboratory of the Swiss Federal Laboratories for Materials Science and Technology (Empa) in St. Gallen. Liu and her team are exploring the unique capabilities of microfluidic GoC models, which can mimic the pathophysiological complexity of glioblastoma, including its interactions with the blood-brain barrier (BBB) and tumor vascularization.
“Glioma is a highly aggressive and complex tumor,” Liu explains. “Traditional 2D and 3D culture models, as well as animal models, have limitations in recapitulating the tumor microenvironment and evaluating treatment effectiveness. Microfluidic GoC models offer a more dynamic and physiologically relevant platform for studying glioma and testing potential therapies.”
The review critically examines current glioma modeling platforms, highlighting the advantages of microfluidic devices. These models can simulate the intricate interactions between tumor cells and their microenvironment, providing valuable insights into drug permeability, tumor progression, and therapeutic responses.
“This technology allows us to create a more accurate representation of the tumor’s behavior in the body,” Liu says. “By understanding these interactions, we can develop more targeted and effective treatments for glioma patients.”
The potential clinical impacts of this research are significant. Improved preclinical models can accelerate the development of new therapies and reduce the time and cost associated with drug discovery. For the energy sector, this technology could also have commercial implications, as it may lead to the development of more efficient and targeted drug delivery systems, reducing the need for invasive procedures and improving patient outcomes.
As the field continues to evolve, the review establishes key criteria for designing robust and clinically relevant glioma models. By addressing these challenges and highlighting future opportunities, the critical role of GoC platforms in advancing glioma research is emphasized.
“This is an exciting time for the field,” Liu concludes. “With continued advancements in organ-on-a-chip technology, we are optimistic about the potential for more personalized and effective treatments for glioma and other complex diseases.”
The review, published in ‘Kleine Wissenschaft’ (Small Science), offers a comprehensive overview of the current state of glioma modeling and the promising future of microfluidic GoC models. As researchers continue to push the boundaries of this technology, the potential for transformative impacts on clinical outcomes and the broader healthcare landscape becomes increasingly clear.