In the relentless battle against periodontal disease, a team of researchers led by Yuhan Liu from the Department of Stomatology at the Aviation General Hospital in Beijing, China, has made a significant stride. Their work, published in the journal *Nano Select* (translated to English as “Nano Choice”), introduces a novel biomaterial that could revolutionize alveolar bone regeneration. The material, zinc-doped hydroxyapatite nanorods (Zn-HAp NRs), not only promotes bone growth but also fights infection, offering a dual-function solution to a persistent medical challenge.
Periodontal disease is a widespread issue, often leading to the deterioration of alveolar bone and eventual tooth loss. Traditional treatments have struggled to effectively regenerate bone tissue while simultaneously combating infection. Liu and his team aimed to address this gap by developing a material that could do both. “Our goal was to create a biomaterial that not only enhances bone regeneration but also possesses antibacterial properties,” Liu explained. “This dual functionality is crucial for effective periodontal treatment.”
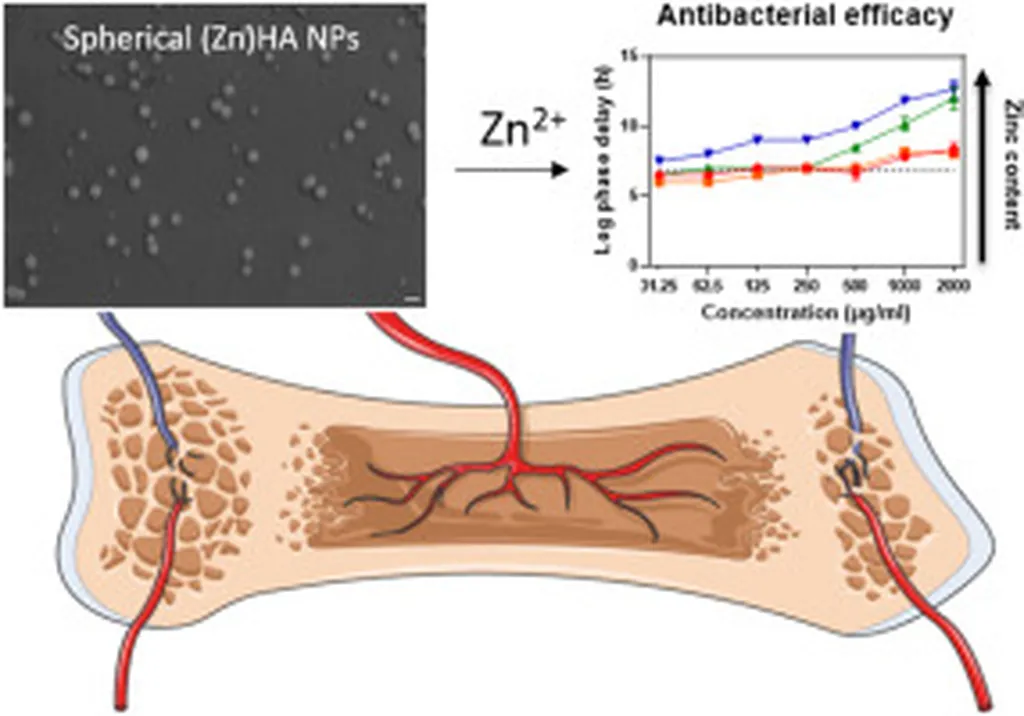
The researchers synthesized Zn-HAp NRs using a one-step in situ mineralization process, a method that is not only efficient but also scalable. In vitro experiments demonstrated that the nanorods significantly promoted osteogenic differentiation, meaning they encouraged the formation of bone cells. Moreover, the material exhibited notable antibacterial properties, potentially reducing the risk of infection during the healing process.
The implications of this research are substantial. For the dental and medical industries, Zn-HAp NRs could offer a more effective and biocompatible solution for alveolar bone regeneration. The material’s dual functionality could lead to better patient outcomes and reduced treatment times, ultimately lowering healthcare costs.
Beyond the immediate medical applications, this research could also pave the way for advancements in other areas of regenerative medicine. The one-step synthesis process, for instance, could be adapted for the production of other biomaterials, making it a valuable innovation for the broader field.
However, Liu cautions that while the in vitro results are promising, further in vivo studies are necessary to validate the material’s efficacy and safety in the complex periodontal microenvironment. “We are optimistic about the potential of Zn-HAp NRs, but we must ensure their safety and effectiveness in real-world applications,” he said.
As the research progresses, the dental and medical communities will be watching closely. The development of Zn-HAp NRs represents a significant step forward in the fight against periodontal disease, offering hope for more effective treatments and better patient outcomes. With further validation, this innovative biomaterial could become a cornerstone of periodontal regeneration, shaping the future of dental care.