In a significant stride towards bridging the efficiency gap in hydrogen production, researchers have developed a novel electrocatalyst that dramatically improves the hydrogen evolution reaction (HER) in alkaline conditions. This breakthrough, led by Ashwani Kumar of the Department of Heterogeneous Catalysis at the Max-Planck-Institut für Kohlenforschung in Germany, could reshape the energy sector by making alkaline electrolysis more viable for hydrogen production.
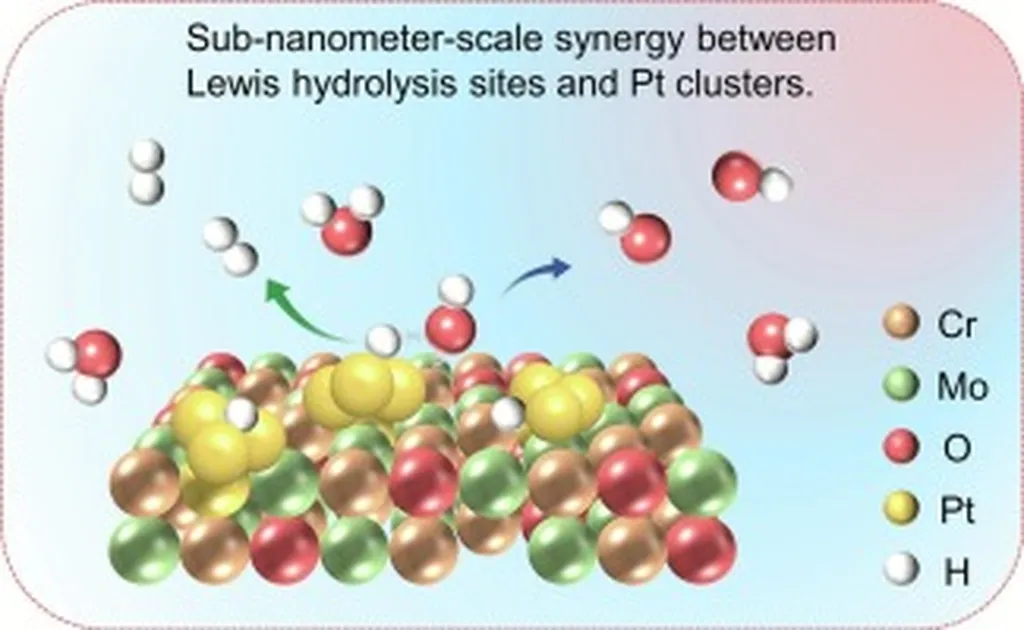
The study, published in *Small Science* (which translates to “Small Science” in English), focuses on the sluggish kinetics of HER in alkaline media, a longstanding challenge due to the additional water dissociation step required. Traditional metal-supported electrocatalysts have shown promise by leveraging hydrogen spillover, but designing highly active catalysts has remained elusive. Kumar and his team tackled this by fabricating platinum nanoclusters (PtNC) on oxygen-defect-rich nickel oxide (NiO) nanowires (PtNC-D-NiO).
The resulting electrocatalyst demonstrated exceptional intrinsic and mass-normalized HER activity, outperforming both PtNC on pristine NiO nanowires and commercial platinum on carbon (Pt/C). Notably, its alkaline HER activity closely matched its acidic counterpart, significantly narrowing the activity gap compared to commercial Pt/C. “This is a substantial advancement,” Kumar explained. “By enhancing proton spillover at the Pt-cluster/NiO interface, we’ve facilitated rapid water dissociation and improved local hydrogen coverage on the PtNC, accelerating the subsequent hydrogen recombination.”
The team’s advanced ex situ and operando physicochemical characterizations, including in situ electrochemical impedance spectroscopy, revealed that oxygen defects substantially lower the water dissociation energy barrier. This facilitates rapid hydrogen spillover and enhances local hydrogen coverage on PtNC, thus boosting alkaline HER performance.
The implications for the energy sector are profound. Alkaline electrolysis, which is generally safer and more cost-effective than acidic electrolysis, has often been hampered by its lower efficiency. This research could make alkaline electrolysis a more competitive option for hydrogen production, potentially reducing costs and improving scalability. “Our work not only offers a cost-effective catalyst design strategy but also provides fundamental insights into the role of hydrogen spillover in optimizing electrocatalytic performance,” Kumar added.
As the world increasingly turns to hydrogen as a clean energy carrier, this breakthrough could accelerate the adoption of alkaline electrolysis technologies. By narrowing the activity gap between acidic and alkaline conditions, the research paves the way for more efficient and economical hydrogen production, ultimately contributing to a more sustainable energy future. The study’s findings, published in *Small Science*, highlight the critical role of advanced materials and fundamental research in driving technological innovation.